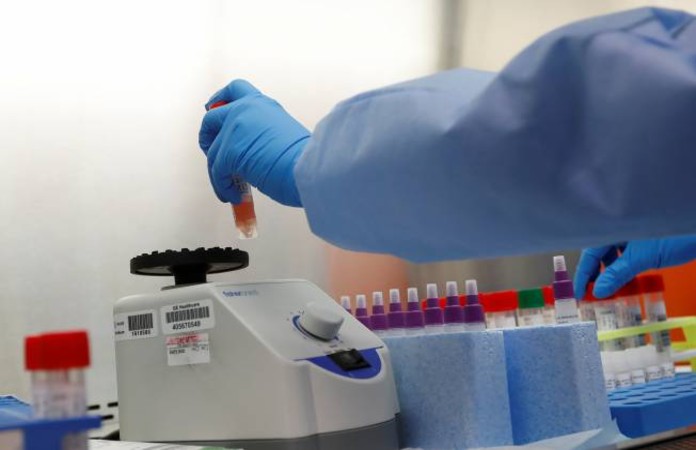
Pune-based molecular diagnostics company Mylab Discovery Solutions Pvt Ltd, which specialises in molecular diagnostic kits has developed first made-in-India test kit for COVID-19 in a record time of six weeks.
It is the first one to receive commercial approval from the Indian FDA/ Central Drugs Standard Control Organisation (CDSCO). Besides, Mylab is the only Indian company to have achieved 100 per cent sensitivity and specificity in the ICMR evaluation.
RT-PCR kits can study 1000 samples from large labs and 200 from smaller labs. Mylab expects to produce one lakh test kits per week and then gradually increase the production to meet the testing demands.
Mylab medical director Dr Gautam Wankhde said: “In the current lab-based testing, it takes four years for test results to come out, Mylab’s turnaround time is two and half hours.” The kits are expected to be priced approximately INR 1200.
“With emphasis on ‘Make in India’ and support from local and central government, the COVID- 19 kit, has been made as per WHO/CDC guidelines. It was developed and evaluated in a record time,” Hasmukh Rawal, Managing Director, Mylab Discovery Solutions, said.
Mylab also manufactures diagnostic detection kits for determining an individual’s mutation status and offers products under the ‘OncoScreen’ brand name.
India faced a lot of backlash for testing fewer people for the Coronavirus. By testing 15 people per million, it is testing the lowest number of people in the world.
The Indian Council of Medical Research initially tested only those people who had travel history and showed symptoms. Later, to expand testing, ICMR roped in 12 more private labs to increase testing. However, these private labs lack approved testing kits that are necessary.